TGA granted approval for the doses of the University of Oxford/AstraZeneca vaccine manufactured in Melbourne by biotechnology company CSL…reports Asian Lite News
The Therapeutic Goods Administration (TGA) announced the approval of the doses of the University of Oxford/AstraZeneca vaccine manufactured in Melbourne by biotechnology company CSL
Australia’s medical regulator on Monday approved the domestically-produced AstraZeneca coronavirus vaccine.
The Therapeutic Goods Administration (TGA) announced the approval of the doses of the University of Oxford/AstraZeneca vaccine manufactured in Melbourne by biotechnology company CSL, reports Xinhua news agency.
In a statement it described the approval as a “critical and very exciting milestone” in Australia’s pandemic response.
“Specific approval of Australian manufacturing by TGA was required to ensure that the locally-manufactured vaccine had exactly the same composition and performance as overseas-manufactured vaccine, was made to the same quality and is free of contaminants,” it said.
Australia has agreed to acquire 53.8 million doses of the AstraZeneca vaccine, 50 million of which will be produced by CSL.
Also read:Covid-19:Australia to allocate more funds
The TGA said that the first domestically-produced vaccines were expected to be released “in the next few days”.
Greg Hunt, the minister for health, said it was a “critical next step”.
“It means the manufacturing process for CSL has been approved, it’s safe, it’s effective,” he told the Australian Broadcasting Corporation (ABC).
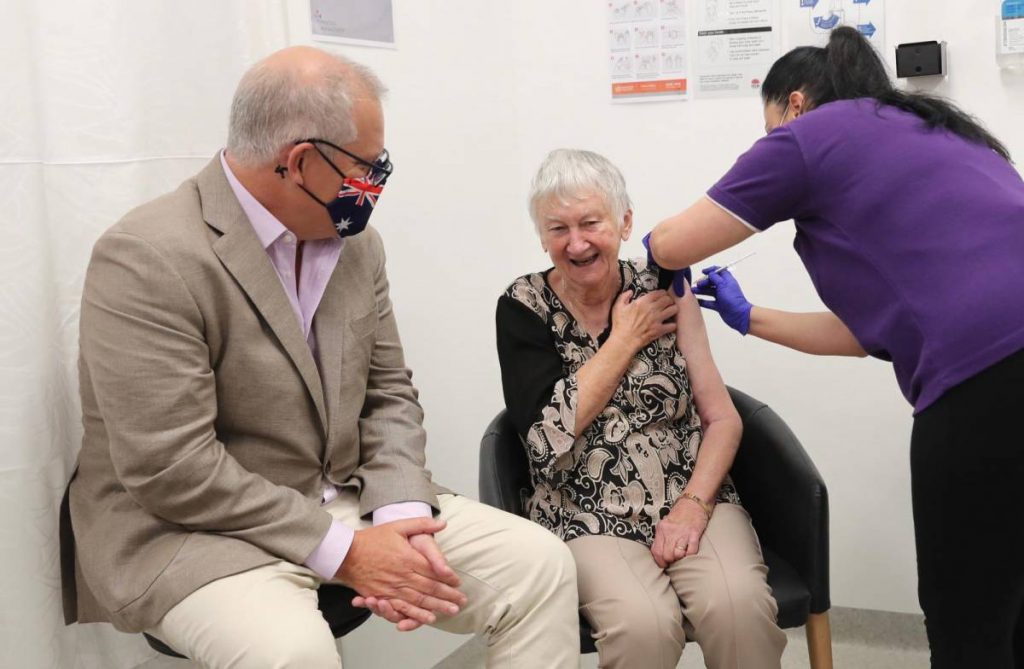
The approval is expected to significantly accelerate the speed of the vaccine rollout in Australia.
More than 6 million Australians became eligible to receive vaccines from Monday under phase 1B of the rollout, which covers the elderly, critical workers and people with underlying health conditions.
Australia has so far reported 29,196 confirmed Covid-19 cases, while the death toll stood at 909.
Also read:Popularity of Australian govt declines













