
Announcement came days after several states have been complaining about shortage of vaccines, reports Asian Lite News
With several states urging the Centre to share the formula of Covid-19 vaccines with other manufacturers to increase production, NITI Aayog member Dr. VK Paul has informed that ‘Bharat Biotech has welcomed this.’
Dr. VK Paul said, “People say that Covaxin be given to other companies for manufacturing. I am happy to say that Covaxin manufacturing company (Bharat Biotech) has welcomed this when we discussed it with them. Under this vaccine live virus is inactivated and this is done only in BSL3 labs. Not every company has this. We give an open invitation to companies who want to do this. Companies that want to manufacture Covaxin, should do it together. Govt will assist so that capacity is increased,” he said.
The announcement came days after several states have been complaining about shortage of vaccines for eligible candidates.
DCGI nod for clinical trial of Covaxin on 2 to 18-years-old
Meanwhile, the Drugs Controller General of India (DCGI) has accorded permission to conduct the Phase II-III clinical trial of Covaxin in the age group of two to 18-years-old to its manufacturer Bharat Biotech Ltd, Union Health Ministry has said.
“The trial will be conducted in 525 healthy volunteers,” said the Ministry.
In the trial, the Ministry said, the vaccine will be given by intramuscular route in two doses at day 0 and day 28.
After careful examination, the DCGI accepted the recommendation of Subject Expert Committee (SEC). The national drug regulator of the country on Wednesday gave the approval to conduct the clinical trial in the age group of youngest age group till now paving way for them to be saved from the deadly pandemic outbreak.
Hyderabad-based Bharat Biotech International Ltd (BBIL) had proposed to carry out a Phase II-III clinical trial of Covaxin in the age group of two to 18 years.
As rapid regulatory response, the proposal was deliberated in Subject Expert Committee (SEC) (Covid-19) on May 11 this year.
The committee after detailed deliberation recommended for grant of permission to conduct proposed Phase II-III clinical trial to certain conditions.
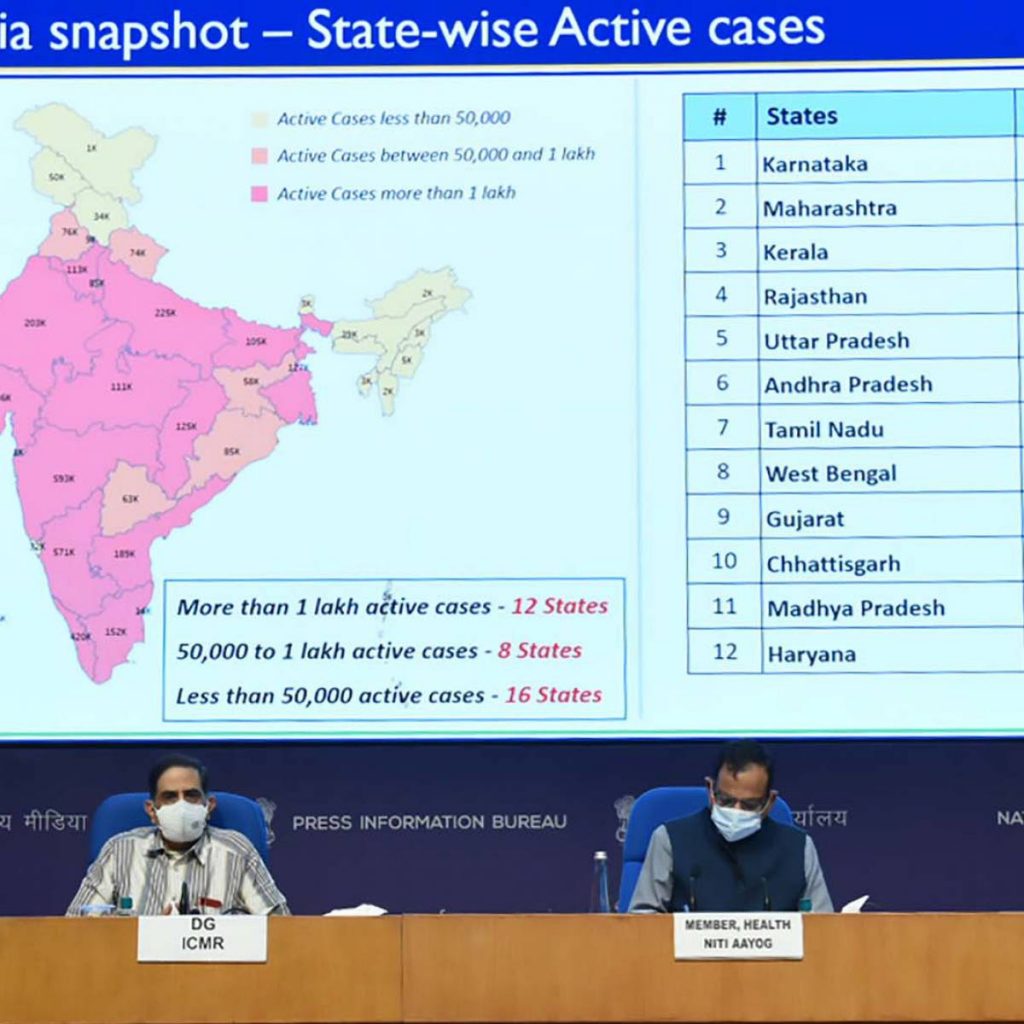
India reports 3,43,144 new coronavirus cases
With 3,43,144 people testing positive for coronavirus in a day, India’s Covid-19 tally of cases climbed to 2,40,46,809, while the death toll rose to 2,62,317 with 4,000 fresh fatalities, according to the Union Health Ministry data updated on Friday.
The active cases have reduced to 37,04,893 comprising 15.41 per cent of the total infections. The national Covid-19 recovery rate has improved to 83.50 per cent, the data updated at 8 am showed.
The number of people who have recuperated from the disease surged to 2,00,79,599, while the case fatality rate was recorded at 1.09 per cent, the data stated.
The 4,000 new fatalities include 850 from Maharashtra, 344 from Karnataka, 308 from Delhi, 297 from Tamil Nadu, 277 from Uttar Pradesh, 186 from Punjab, 195 from Chhattisgarh, 163 from Haryana, 159 from Rajasthan, 129 from West Bengal, 122 from Uttarakhand, 109 from Gujarat and 108 from Jharkhand.
A total of 2,62,317 deaths have been reported so far in the country including 78,857 from Maharashtra, 20,712 from Karnataka, 20,618 from Delhi, 16,768 from Tamil Nadu, 16,646 from Uttar Pradesh, 12,857 from West Bengal, 11,297 from Punjab and 11,289, from Chhattisgarh.
ALSO READ-Pakistan to produce China’s vaccine
READ MORE-India to produce 9 crore vaccine doses by June


